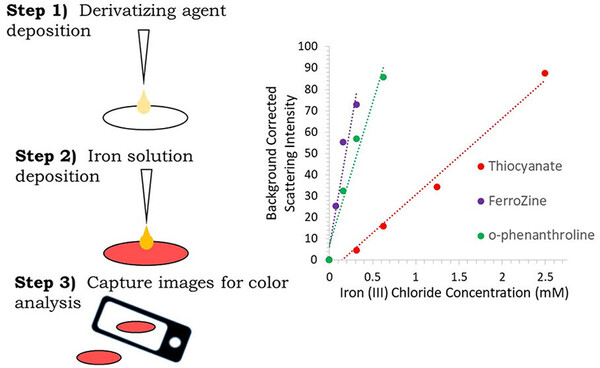
Fentanyl test strips have "blind spots" for detection of fentanyl analogs--Kat's paper is now published!
Fentanyl test strips are an important tool for detecting opioids that can cause fatal overdoses.
By testing over 200 fentanyl analogs with two brands of fentanyl test strips (FTS), Kathleen Hayes found that the test strips can detect many, but not all, fentanyl analogs. The types of analogs…
 …
…